VALIDATION, UNCERTAINTY AND
QUALITY CONTROL
QUALITY CONTROL
ConfLab Validation
The ConfLab Validation software performs many calculations, considering different validation parameters, focusing on the compliance of references for method validation and the new ISO 17025: 2017. The idea is that the user only enters with the experimental values and the software, automatically, calculates the parameters, presents the results and allows the printing of a fully customizable validation report, with all the traceability data required by the quality management systems . It is also compatible and meets the requirements of Good Laboratory Practices (GLP). Among the parameters for the Validation of Methods, is highlighted:
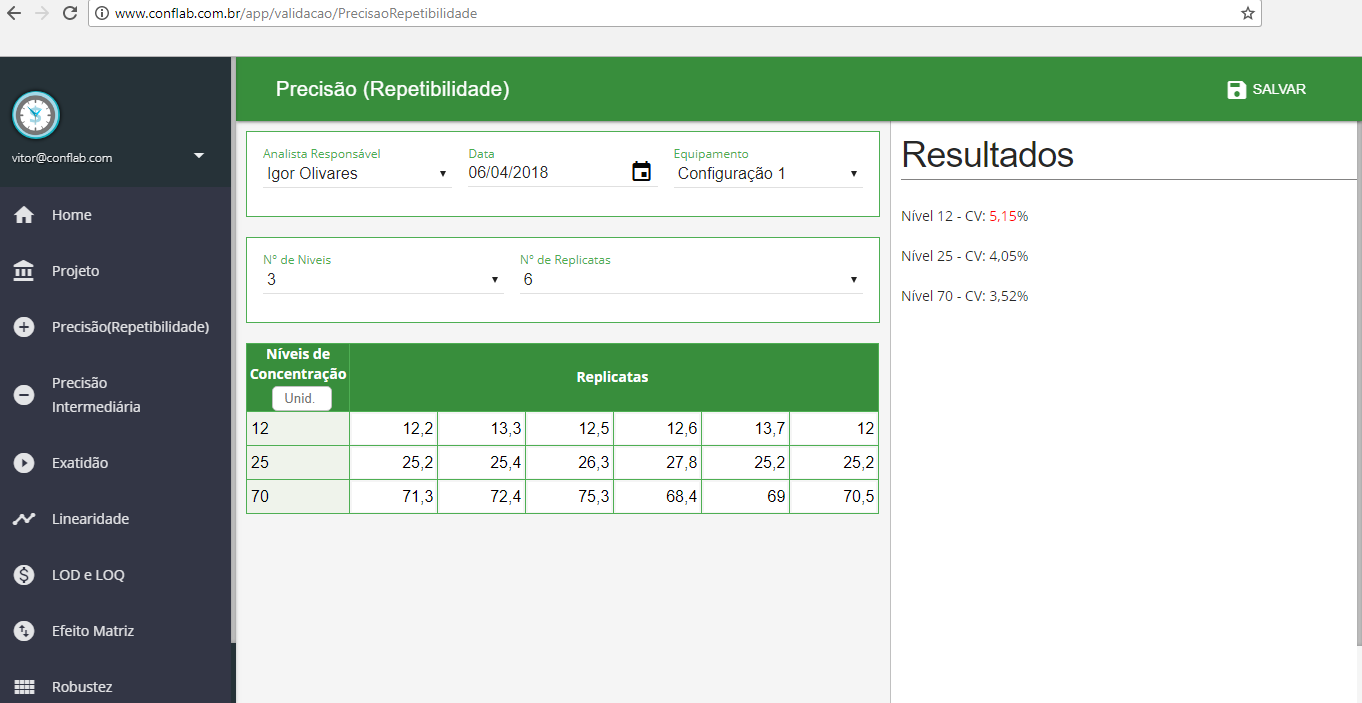
- Precision (Repeatability)
- Intermediate Precision
-
Accuracy
- Accuracy with Fortified Samples
- Accuracy with Use of Reference Material
-
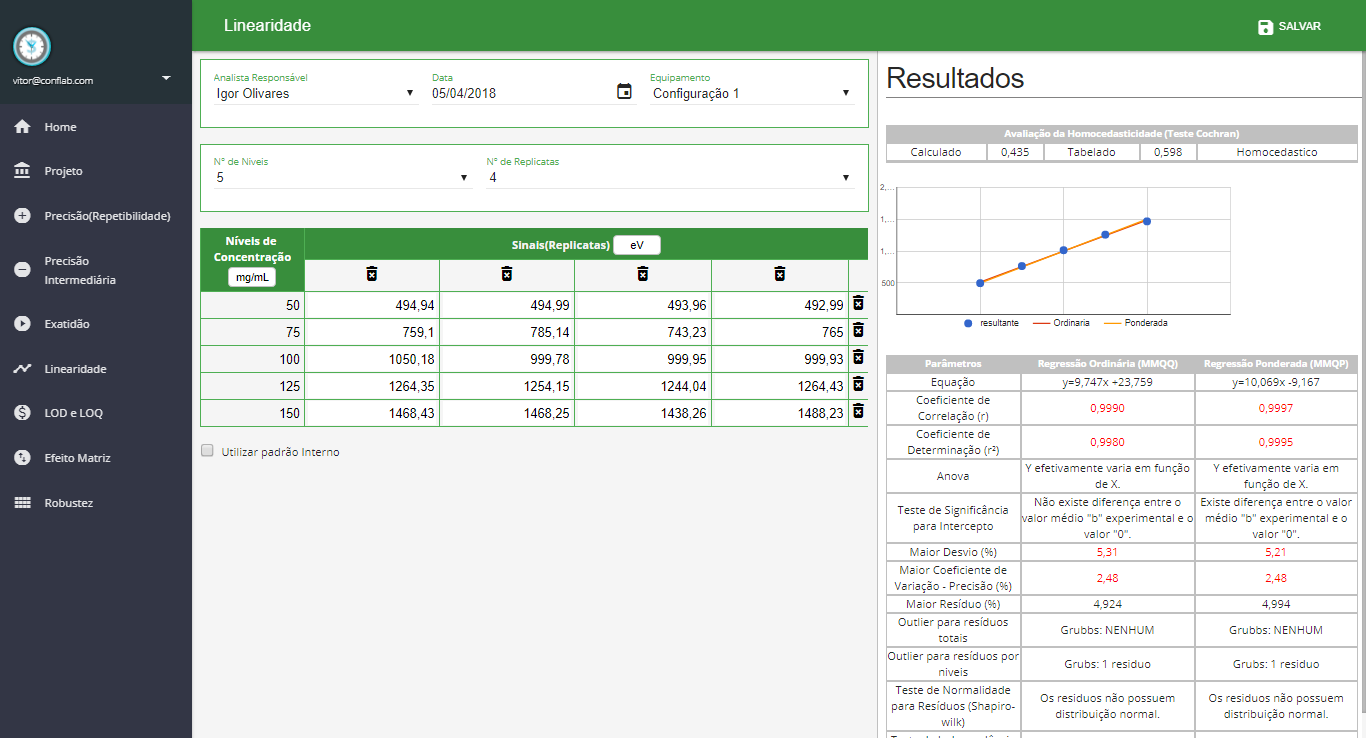
Linearity
- Homoscedasticity Evaluation
-
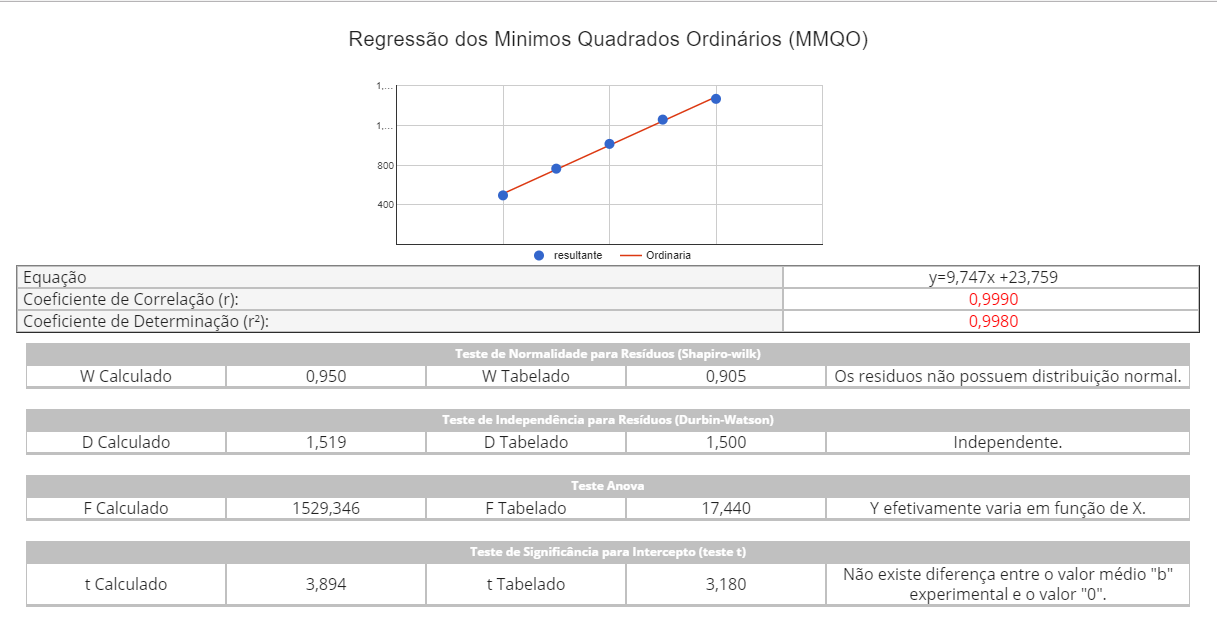
Regression of Ordinary Least Squares (MMQO) and Weighted Least Squares Regression (MMQP)
- General Testing
- Normality
- Independence
- Anova
- Significance of Intercept
- Absolute residue
- Relative residue
- Outliers (Grubbs by Level)
- Outliers (Total Grubbs)
- Accuracy
- Coefficient of variation
- Regression of Ordinary Least Squares (MMQO) Versus Regression of Weighted Minimum Least Squares (MMQP)
-
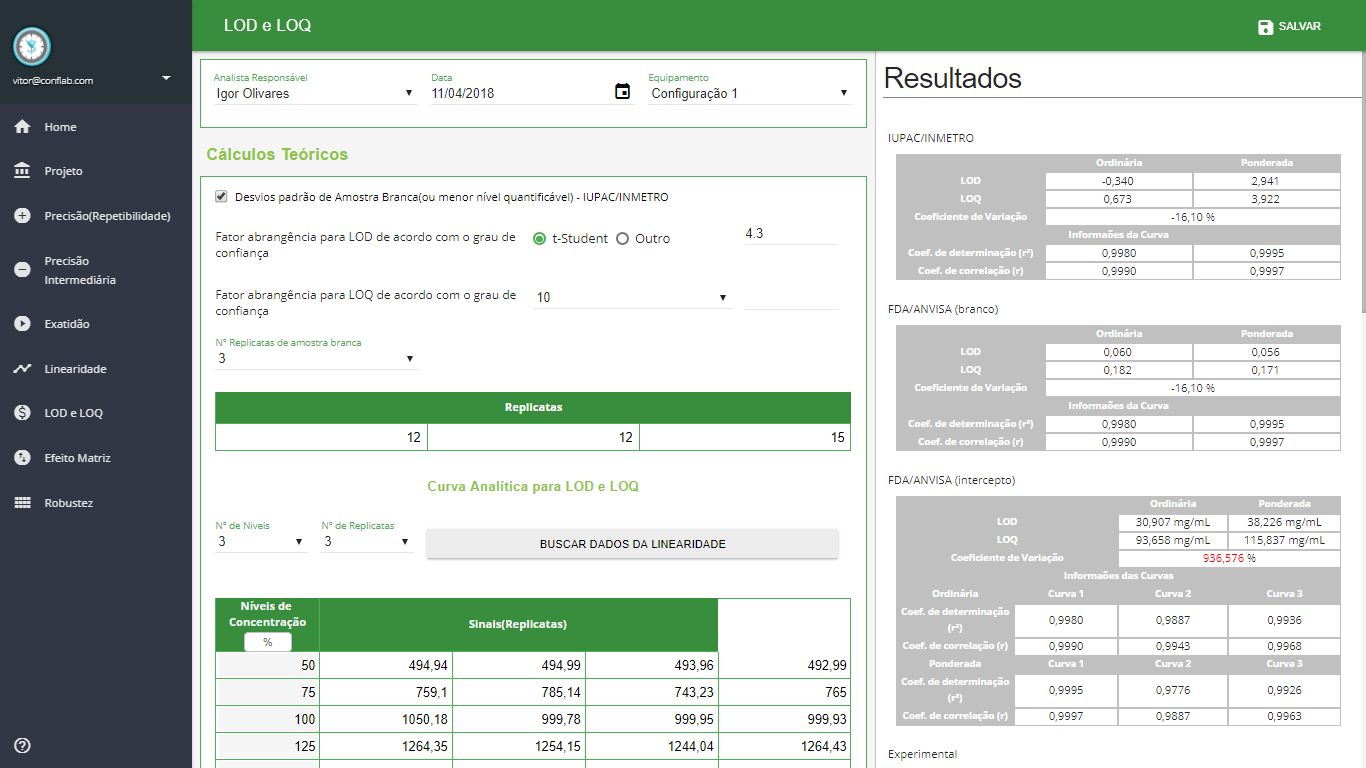
LOD and LOQ
- Theoretical Calculations - White Sample standard deviations (or lower quantifiable level) - IUPAC / INMETRO
- Theoretical Calculations - White Sample standard deviations (or lower quantifiable level) - FDA / ANVISA
- Theoretical Calculations - Standard Deviation of Signal Intercept - FDA / ANVISA
- Experimental Calculations
-
Matrix Effect
- Differences Between Calibration Curves
- Deviations from FMNs
-
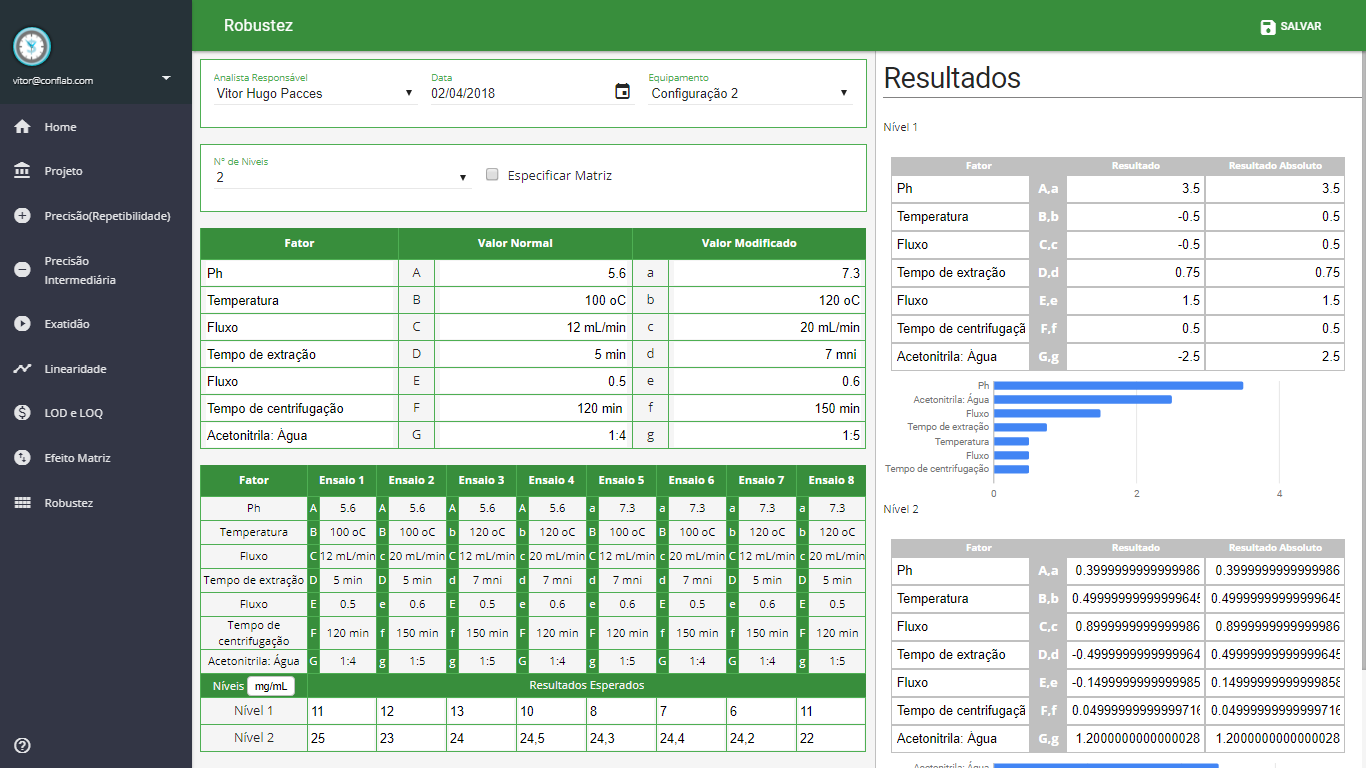
Robustness
- Youden's Test

Validation, uncertainty and quality control
All rights reserved
phone(19) 99176-7122