VALIDATION, UNCERTAINTY AND
QUALITY CONTROL
QUALITY CONTROL
ConfLab Uncertainty
ConfLab Uncertainty Software is based on the concepts defined by the most widely known and applied uncertainty estimation guide for analytical chemistry, the Eurachem / Citac guide [01].
This guide points out that the uncertainty assessment requires the analyst to observe in detail all possible sources of uncertainty. However, while such a detailed study may require considerable effort, it is essential that the applied effort is not disproportionate. This concern is also highlighted by Michael Thompson [2] who states that an undue emphasis on traceability and a particular approach to uncertainty has been counterproductive.
The evaluation of uncertainty requires the analyst to look closely at all the possible sources of uncertainty.
In practice a preliminary study will quickly identify the most significant sources of uncertainty (which can be started by the measurand equation and validation data) and, as the examples show, the value obtained for the combined uncertainty is almost entirely controlled by the major contributions. A good estimate of uncertainty can be made by concentrating effort on the largest contributions. Further, once evaluated for a given method applied in a particular laboratory (i.e. a particular measurement procedure), the uncertainty estimate obtained may be reliably applied to subsequent results obtained by the method in the same laboratory, provided that this is justified by the relevant quality control data. No further effort should be necessary unless the procedure itself or the equipment used is changed, in which case the uncertainty estimate would be reviewed as part of the normal re-validation [01]. This statement is also supported by ISO / IEC 17025: 2017 [03] which highlights in its requirement 7.6.3 (note 2): “For a particular method in which the measurement uncertainty of results has been established and verified, there is no need to evaluate the measurement uncertainty for each result if the laboratory can demonstrate that the influence factors identified as critical are under control. ”
References
[01] Eurachem / Citac Guide Quantifying Uncertainty in Analytical Measurement 3rd Edition.
[02] Anal. Methods, 2014, 6, 8454-8459
[03] ISO / IEC 17025: 2017. General requirements for the competence of testing and calibration laboratories.
[01] Eurachem / Citac Guide Quantifying Uncertainty in Analytical Measurement 3rd Edition.
[02] Anal. Methods, 2014, 6, 8454-8459
[03] ISO / IEC 17025: 2017. General requirements for the competence of testing and calibration laboratories.
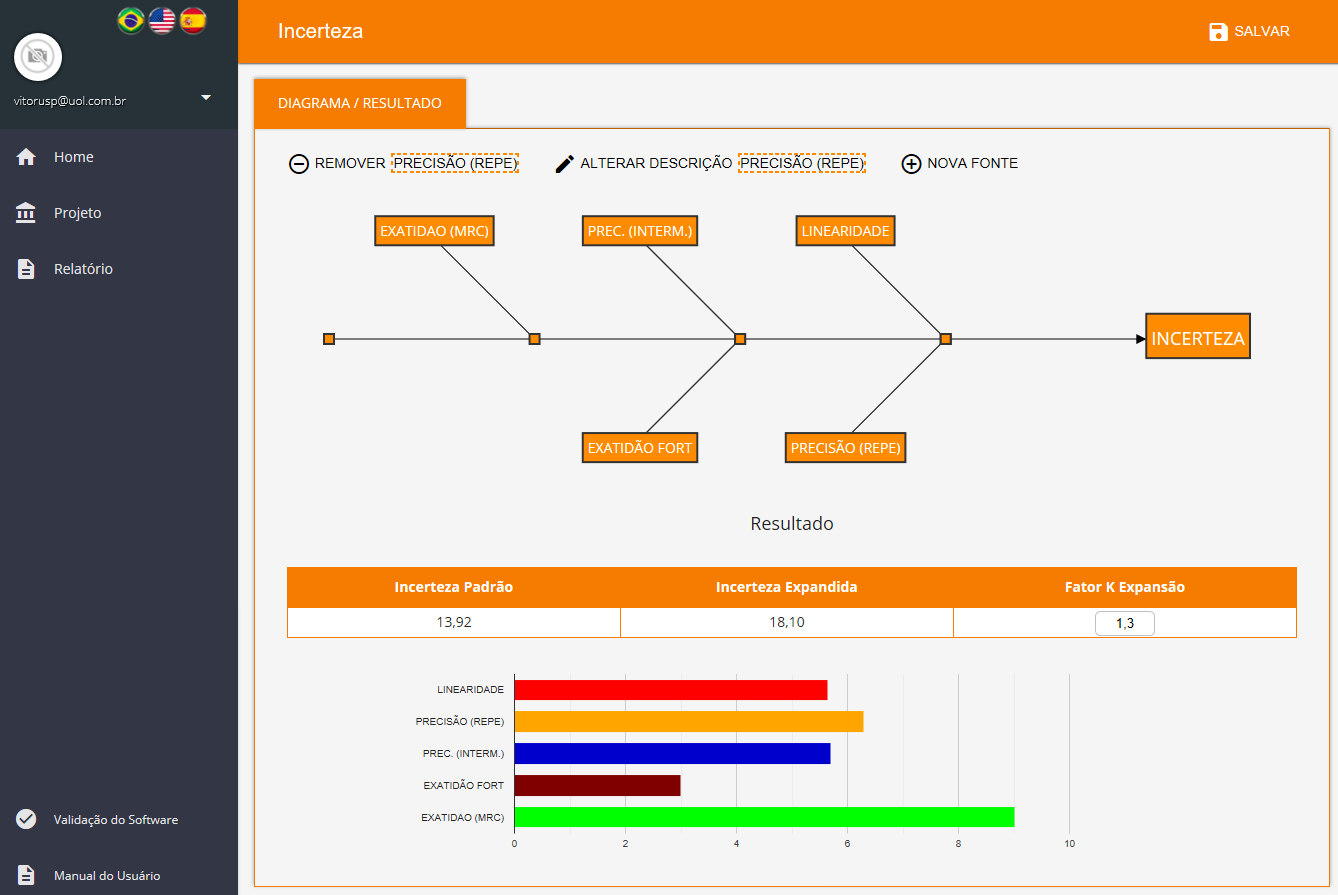
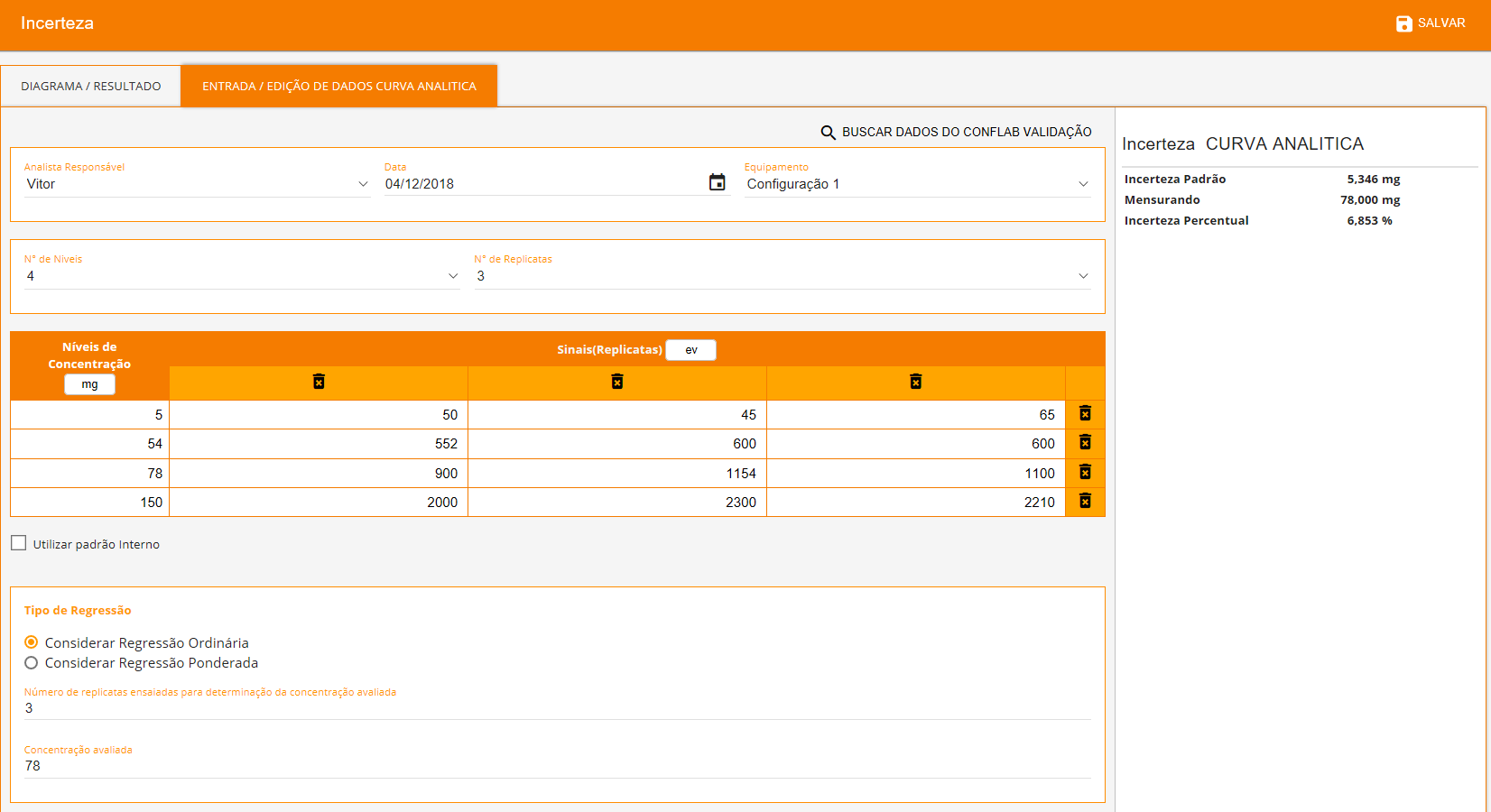
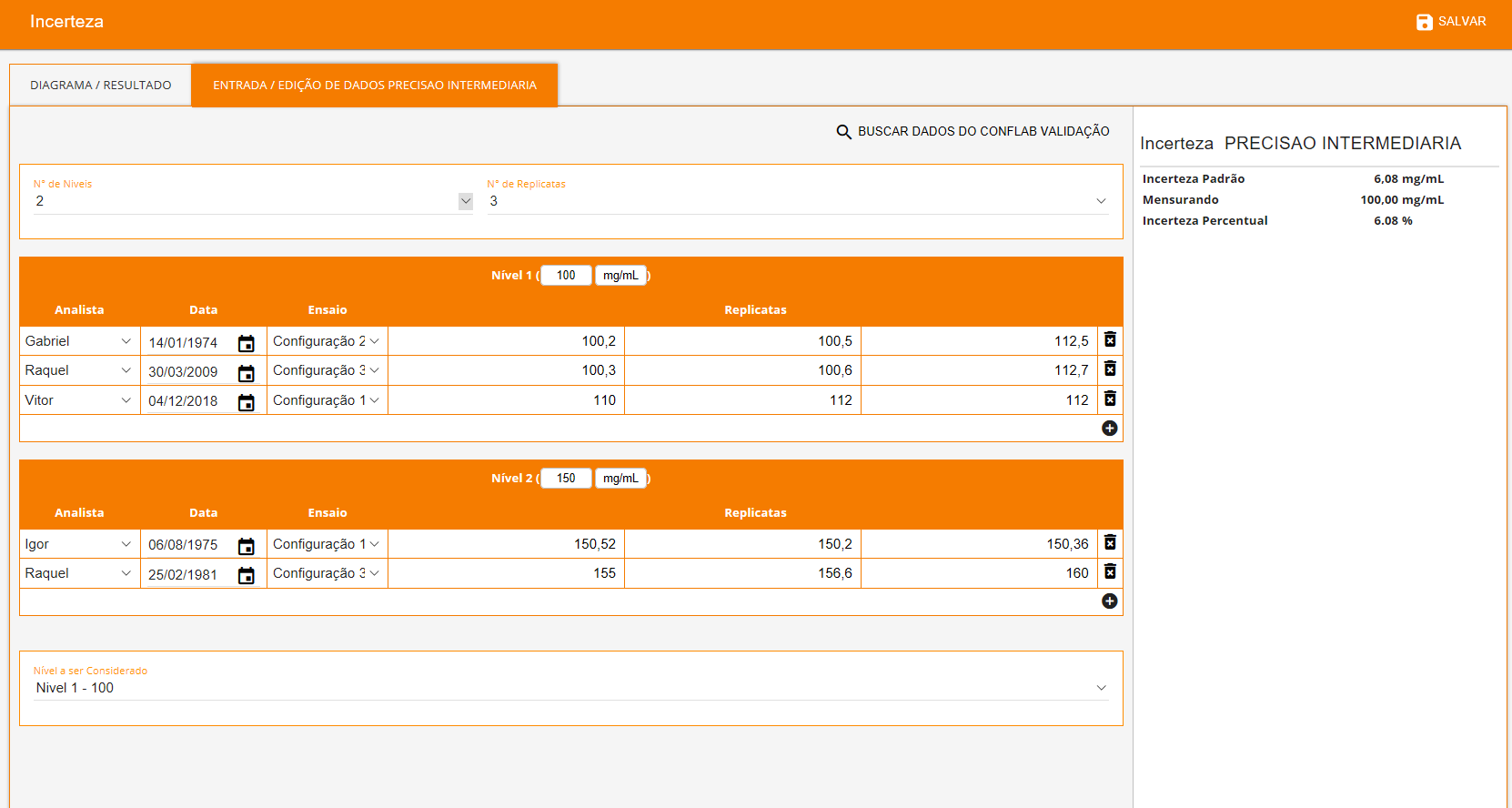
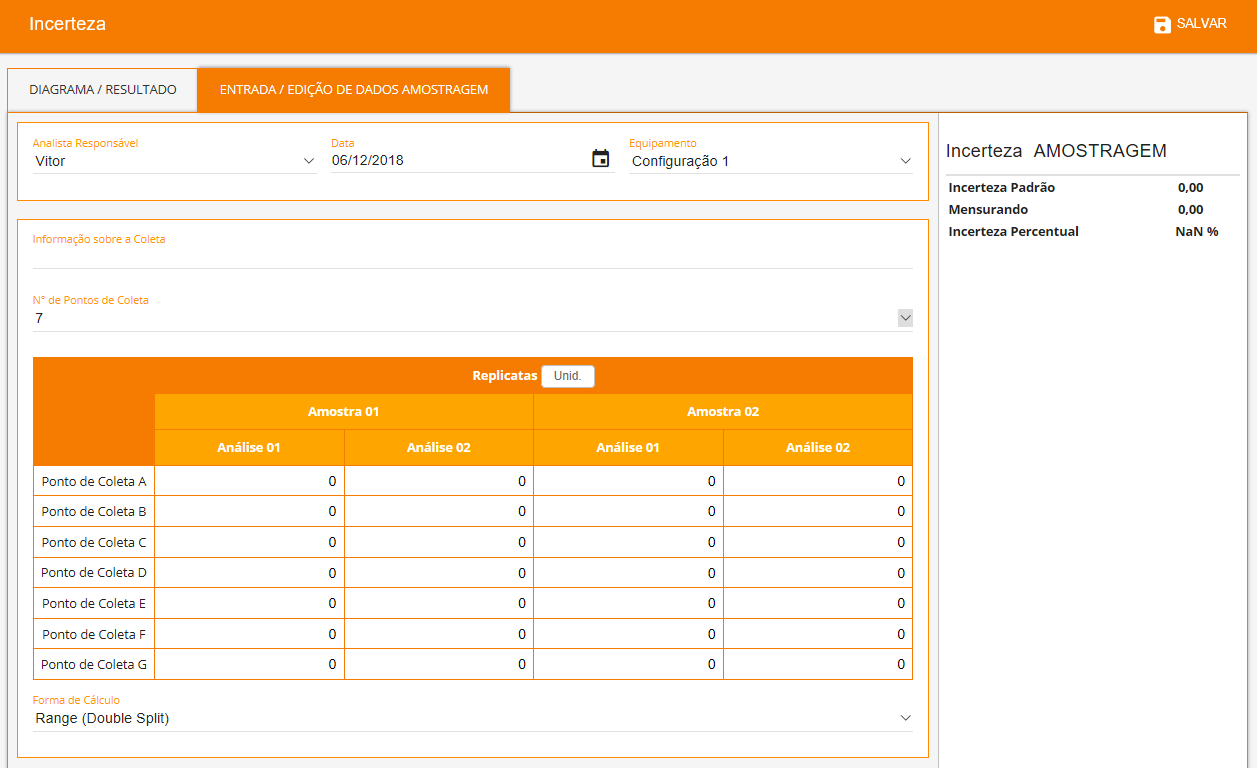
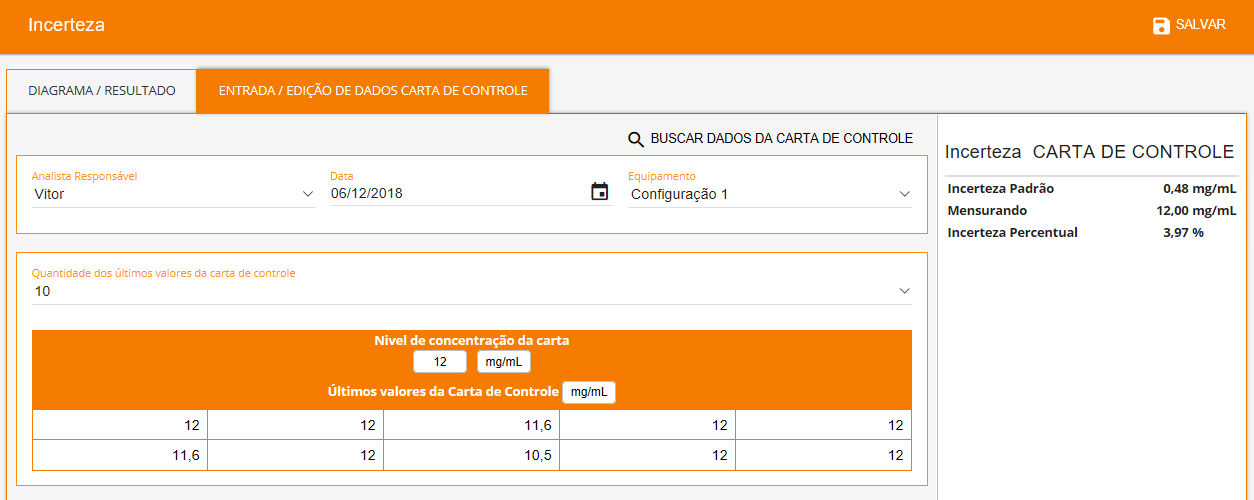
The software allows you to search for validation data for uncertainty estimation, and presents numerous source types that can be chosen to suit the characteristics of each test method as showed below. Each source will be automatically inserted into an Ishikawa diagram and all results will be showed in a Pareto diagram (aiming to evaluate the largest contributions) followed by the final uncertainty.
-
Uncertainty from Linearity
- Ordinary regression model;
- Weighted regression model;
- Uncertainty from Precision (Repeatability)
- Uncertainty from Precision (Intermediate)
- Accuracy Uncertainty (Fortified Samples)
- Accuracy Uncertainty (Use of Reference Material)
- Mass Uncertainty
- Volume Uncertainty
- Uncertainty from Solution Standardization
- Uncertainty from Reference Material
- Uncertainty from Other Sources or Equipment
-
Uncertainty from Sampling
- Anova;
- Range Double Split;
- Uncertainty from Control Chart

Validation, uncertainty and quality control
All rights reserved
phone(19) 99176-7122